Biotinylated Human CXCL12 (SDF-1α)
|
BACKGROUND
Stromal-Cell Derived Factor-1α (SDF-1α) (CXCL12) attracts lymphocytes and plays important roles in embryogenesis and angiogenesis with implications in tumor metastasis. By binding to CXCR4, one of the co-receptors for HIV viral entry, SDF-1α can suppress HIV. Besides CXCR4, SDF-1 α also binds to the “decoy” receptor CXCR7, and activates the downstream β-arrestin- rather than G protein-mediated signaling pathway.
Biotinylated CXCL12 is made using an enzymatic method, which has several advantages over chemical biotinylation methods. The attachment of biotin at a specific lysine residue is nearly 100% complete, and leads to a modified chemokine with functionalities comparable to those of the unmodified CXCL12 in calcium flux and migration assay. Combining with avidin analogues conjugated to various fluorescent labels, biotinylated CXCL12 is useful in studies on receptor identification, distribution, chemokine binding, and other cellular assays. They serve as great tools in visualization and quantification, and replace the needs for radioactively labeled chemokines.
Stromal-Cell Derived Factor-1α (SDF-1α) (CXCL12) attracts lymphocytes and plays important roles in embryogenesis and angiogenesis with implications in tumor metastasis. By binding to CXCR4, one of the co-receptors for HIV viral entry, SDF-1α can suppress HIV. Besides CXCR4, SDF-1 α also binds to the “decoy” receptor CXCR7, and activates the downstream β-arrestin- rather than G protein-mediated signaling pathway.
Biotinylated CXCL12 is made using an enzymatic method, which has several advantages over chemical biotinylation methods. The attachment of biotin at a specific lysine residue is nearly 100% complete, and leads to a modified chemokine with functionalities comparable to those of the unmodified CXCL12 in calcium flux and migration assay. Combining with avidin analogues conjugated to various fluorescent labels, biotinylated CXCL12 is useful in studies on receptor identification, distribution, chemokine binding, and other cellular assays. They serve as great tools in visualization and quantification, and replace the needs for radioactively labeled chemokines.
|
SPECIFICATIONS
Source: E. coli derived Accession # P48061-2 (22-89) Modification: Biotin at C-Terminal Formulation: Lyophilized Carrier Protein: None Predicted Molecular Mass: 10.381kDa Extinction Coefficient: 14,180 M-1 cm-1 Actual Molecular Mass: 10.381kDa by ESI Mass Spec Endotoxin Level: <0.01 EU per 1μg of the protein by the LAL method Purity: > 97% by SDS PAGE Flow Cytometry
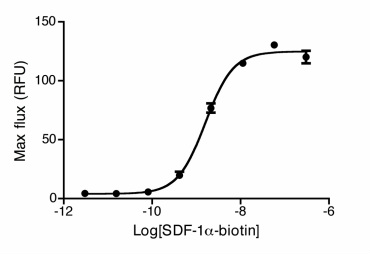
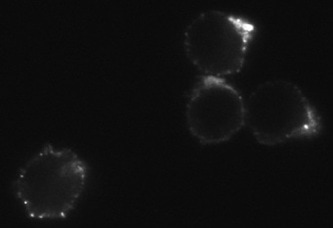
Uptake of 20nM CXCL12-biotin by U937 cells in the presence (red trace) and absence (cyan) of a CXCR4 inhibitor, AMD 3100. U937 cells are not stained by streptavidin-PE in the absence of CXCL12-biotin (orange). Flow Cytometry Protocol Dose Dependent Ca2+ Flux HEK293 cells expressing CXCR4 were stimulated with SDF-1α-biotinylated. Calcium flux was measured with calcium dye from Molecular Devices. A dose response curve was obtained and an EC50 of 3.5nM was recorded. |
PREPARATION AND STORAGE
Reconstitution: Spin sample prior to reconstitution. Recommended at 100μg/mL in sterile water Shipping: Room Temp Stability and Storage: Avoid repeated freeze-thaw cycles • 12 months from date of receipt, -20 to -70 °C as supplied. • ChemoTactics suggests using immediately after reconstitution. • 1 month, -20 to -70 °C under sterile conditions after reconstitution. Migration induced by SDF-1α
Jurkat cells expressing endogenous CXCR4 were assayed for migration through the Transwell bare filter at various concentrations of SDF1α. The response is expressed as the % of total input cells (Blue: wild type; Red: biotinylated). Migration Assay Protocol Activity: EC50 = 0.5-0.9nM determined by migration assay with cells expressing recombinant CXCR4
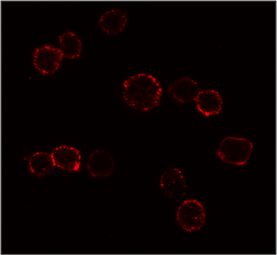
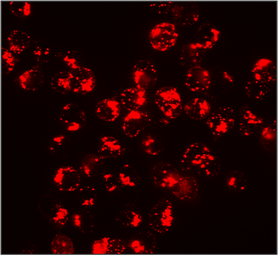
CXCR4 Surface Detection
Confocal microscopy images of surface stained U937 cells with SDF-1α-biotin conjugated to streptavidin-Cy3. |
For bulk orders over 1mg please email us at [email protected] for a quote
|
|
|
|
|
|
|
REFERENCES
1. Pisani, A., Donno, R., Valenti, G., Pompa, P. P., Tirelli, N., & Bardi, G. (2022). Chemokine-decorated nanoparticles target specific subpopulations of primary blood mononuclear leukocytes. Nanomaterials, 12(20), 3560. https://doi.org/10.3390/nano12203560
2. Pisani, A.; Donno, R.; Gennari, A.; Cibecchini, G.; Catalano, F.; Marotta, R.; Pompa, P.P.; Tirelli, N.; Bardi, G. CXCL12-PLGA/Pluronic Nanoparticle Internalization Abrogates CXCR4-Mediated Cell Migration. Nanomaterials 2020, 10, 2304. https://doi.org/10.3390/nano10112304
3. Zhikai Wang, Ran Yan, Jiayun Li, Ya Gao, Philip Moresco, Min Yao, Jaclyn F. Hechtman, Matthew J. Weiss, Tobias Janowitz, Douglas T. Fearon. Pancreatic cancer cells assemble a CXCL12-keratin 19 coating to resist immunotherapy. bioRxiv 776419; doi: https://doi.org/10.1101/776419. Posted: September 4, 2020
4. "A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1)." Bleul. C, Fuhlbrigge. R, Casasnovas, J, Aiuti. A, Springer, T. J. Exp. Med. 184 (3): 1101–9. (1996)
5. "The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry". Bleul. C, Farzan, M., Choe, H., Parolin C., Clark-Lewis I., Sodroski J. Nature 382 (6594): 829–833 (1996)
6. "Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration." Arya, M., Ahmed, H., Silhi, Williamson, M., Patel, H. Tumour Biol. 28 (3): 123–31 (2007)
7. “β-arrestin- but not G protein –mediated signaling by the “decoy” receptor CXCR7.” Rajagopal, S., Kim, J., Ahn, S., Craig, S., lam, C., Gerard, N., Gerard, C., Lefkowitz, R. Proc Natl Acad Sci USA 107(2):628-632 (2010)
1. Pisani, A., Donno, R., Valenti, G., Pompa, P. P., Tirelli, N., & Bardi, G. (2022). Chemokine-decorated nanoparticles target specific subpopulations of primary blood mononuclear leukocytes. Nanomaterials, 12(20), 3560. https://doi.org/10.3390/nano12203560
2. Pisani, A.; Donno, R.; Gennari, A.; Cibecchini, G.; Catalano, F.; Marotta, R.; Pompa, P.P.; Tirelli, N.; Bardi, G. CXCL12-PLGA/Pluronic Nanoparticle Internalization Abrogates CXCR4-Mediated Cell Migration. Nanomaterials 2020, 10, 2304. https://doi.org/10.3390/nano10112304
3. Zhikai Wang, Ran Yan, Jiayun Li, Ya Gao, Philip Moresco, Min Yao, Jaclyn F. Hechtman, Matthew J. Weiss, Tobias Janowitz, Douglas T. Fearon. Pancreatic cancer cells assemble a CXCL12-keratin 19 coating to resist immunotherapy. bioRxiv 776419; doi: https://doi.org/10.1101/776419. Posted: September 4, 2020
4. "A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1)." Bleul. C, Fuhlbrigge. R, Casasnovas, J, Aiuti. A, Springer, T. J. Exp. Med. 184 (3): 1101–9. (1996)
5. "The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry". Bleul. C, Farzan, M., Choe, H., Parolin C., Clark-Lewis I., Sodroski J. Nature 382 (6594): 829–833 (1996)
6. "Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration." Arya, M., Ahmed, H., Silhi, Williamson, M., Patel, H. Tumour Biol. 28 (3): 123–31 (2007)
7. “β-arrestin- but not G protein –mediated signaling by the “decoy” receptor CXCR7.” Rajagopal, S., Kim, J., Ahn, S., Craig, S., lam, C., Gerard, N., Gerard, C., Lefkowitz, R. Proc Natl Acad Sci USA 107(2):628-632 (2010)
Support: [email protected]
For Research use only, Not for use in Humans.
For Research use only, Not for use in Humans.