BACKGROUND
CXCL10 (IP-10) was originally identified as an IFN-gamma-inducible gene in endothelial, fibroblasts and monocytes cells. IP-10 is considered a member of the CXC chemokine subfamily from its protein sequence which includes the four conserved cysteine residues present in CXC chemokines. IP-10 signals through the CXCR3 receptor to selectively attract Th1 lymphocytes and monocytes. It also has angiostatic and mitogenic properties on vascular smooth muscle cells. A diverse population of cell types rapidly increases transcription of mRNA encoding IP-10, which suggests that gamma-induced protein may be a key mediator of the IFNG/IFN-gamma response.
Disease Areas: Auto immune disorders, Cardiovascular disorders, Angiogenesis, Neurobiology
CXCL10 (IP-10) was originally identified as an IFN-gamma-inducible gene in endothelial, fibroblasts and monocytes cells. IP-10 is considered a member of the CXC chemokine subfamily from its protein sequence which includes the four conserved cysteine residues present in CXC chemokines. IP-10 signals through the CXCR3 receptor to selectively attract Th1 lymphocytes and monocytes. It also has angiostatic and mitogenic properties on vascular smooth muscle cells. A diverse population of cell types rapidly increases transcription of mRNA encoding IP-10, which suggests that gamma-induced protein may be a key mediator of the IFNG/IFN-gamma response.
Disease Areas: Auto immune disorders, Cardiovascular disorders, Angiogenesis, Neurobiology
|
SPECIFICATIONS
Source: E. coli derived Accession # P02778 (22-98) Modification: None Formulation: Lyophilized Carrier Protein: None Actual Molecular Mass: 8.6 kDa by ESI Mass Spec Predicted Molecular Mass: 8.6 kDa Extinction Coefficient: 480 M-1 cm-1 (A280), 11 (mg/ml•cm)-1 (A220) Protein Sequence: VPLSRTVRCTCISISNQPVNPRSLEKLEIIPASQFCPRVEIIATMKKKGEKRCLNPESKA IKNLLKAVSKERSKRSP Endotoxin Level: <0.01 EU per 1μg of the protein by the LAL method Purity: > 97% by SDS PAGE PREPARATION AND STORAGE
Reconstitution: Spin sample prior to reconstitution. Recommended at 100μg/mL in sterile water Shipping: Room Temp Stability and Storage: Avoid repeated freeze-thaw cycles • 12 months from date of receipt, -20 to -70 °C as supplied. • 1 month, 2 to 8 °C under sterile conditions after reconstitution. • 3 months, -20 to -70 °C under sterile conditions after reconstitution. |
Migration Assay: Cells expressing recombinant CXCR3 were assayed for migration through a transwell filter at various concentrations of WT IP-10. Responses are expressed as the % of total input cells.
Migration Assay Protocol 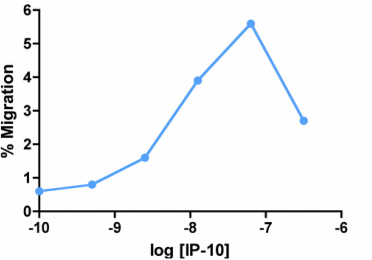
Activity: EC50 = 6.3nM determined by migration assay with cells expressing recombinant CXCR3 receptors
|
For bulk orders or custom sizes, please contact us and we can provide this for you.
|
|
|
|
|
|
REFERENCES
1. "Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins."
Luster A.D., Unkeless J.C., Ravetch J.V. Nature 315:672-676(1985)
2. "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)."
The MGC Project Team. Genome Res. 14:2121-2127(2004)
3. "Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO,
IP-10, and IL-8." Proost P., de Wolf-Peeters C., Conings R., Opdenakker G., Billiau A., van Damme J. J. Immunol. 150:1000-1010(1993)
4. "Human IP-9: a keratinocyte derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3)."
Tensen C.P., Flier J., van der Raaij-Helmer E.M.H., Sampat-Sardjoepersad S., van der Schors R.C., Leurs R., Scheper R.J., Boorsma D.M., Willemze R.
J. Invest. Dermatol. 112:716-722(1999)
5. "Processing of natural and recombinant CXCR3-targeting chemokines and implications for biological activity."
Hensbergen P.J., van der Raaij-Helmer E.M.H., Dijkman R., van der Schors R.C., Werner-Felmayer G., Boorsma D.M., Scheper R.J., Willemze R., Tensen C.P.
Eur. J. Biochem. 268:4992-4999(2001)
6. "Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation."
Loos T., Mortier A., Gouwy M., Ronsse I., Put W., Lenaerts J.P., Van Damme J., Proost P. Blood 112:2648-2656(2008)
7. "The CXCR3 binding chemokine IP-10/CXCL10: structure and receptor interactions."
Booth V., Keizer D.W., Kamphuis M.B., Clark-Lewis I., Sykes B.D. Biochemistry 41:10418-10425(2002)
1. "Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins."
Luster A.D., Unkeless J.C., Ravetch J.V. Nature 315:672-676(1985)
2. "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)."
The MGC Project Team. Genome Res. 14:2121-2127(2004)
3. "Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO,
IP-10, and IL-8." Proost P., de Wolf-Peeters C., Conings R., Opdenakker G., Billiau A., van Damme J. J. Immunol. 150:1000-1010(1993)
4. "Human IP-9: a keratinocyte derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3)."
Tensen C.P., Flier J., van der Raaij-Helmer E.M.H., Sampat-Sardjoepersad S., van der Schors R.C., Leurs R., Scheper R.J., Boorsma D.M., Willemze R.
J. Invest. Dermatol. 112:716-722(1999)
5. "Processing of natural and recombinant CXCR3-targeting chemokines and implications for biological activity."
Hensbergen P.J., van der Raaij-Helmer E.M.H., Dijkman R., van der Schors R.C., Werner-Felmayer G., Boorsma D.M., Scheper R.J., Willemze R., Tensen C.P.
Eur. J. Biochem. 268:4992-4999(2001)
6. "Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation."
Loos T., Mortier A., Gouwy M., Ronsse I., Put W., Lenaerts J.P., Van Damme J., Proost P. Blood 112:2648-2656(2008)
7. "The CXCR3 binding chemokine IP-10/CXCL10: structure and receptor interactions."
Booth V., Keizer D.W., Kamphuis M.B., Clark-Lewis I., Sykes B.D. Biochemistry 41:10418-10425(2002)



